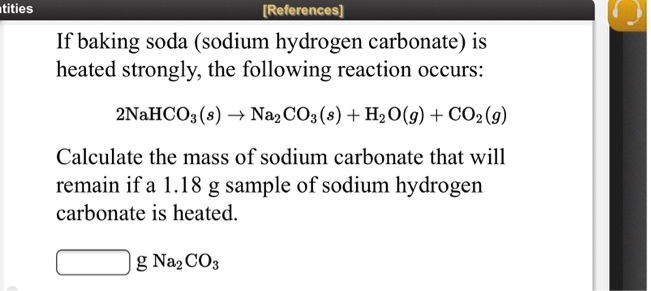
SOLVED: If baking soda (sodium hydrogen carbonate) is heated strongly, the following reaction occurs: 2NaHCO3 (s) â†' Na2CO3 (s) + H2O(g) + CO2(g) Calculate the mass of sodium carbonate that will remain
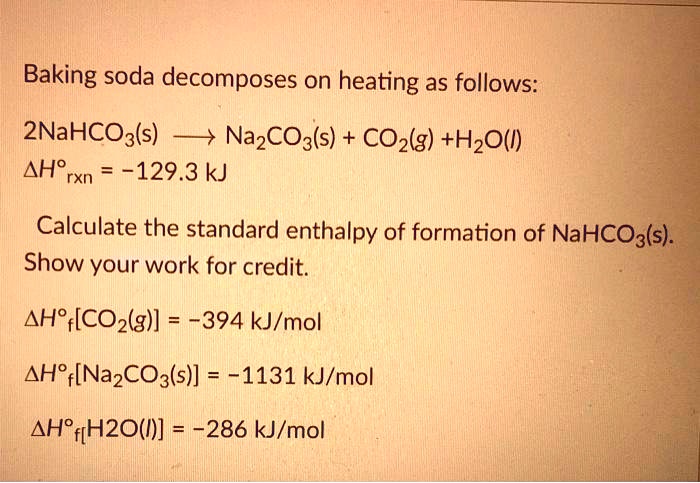
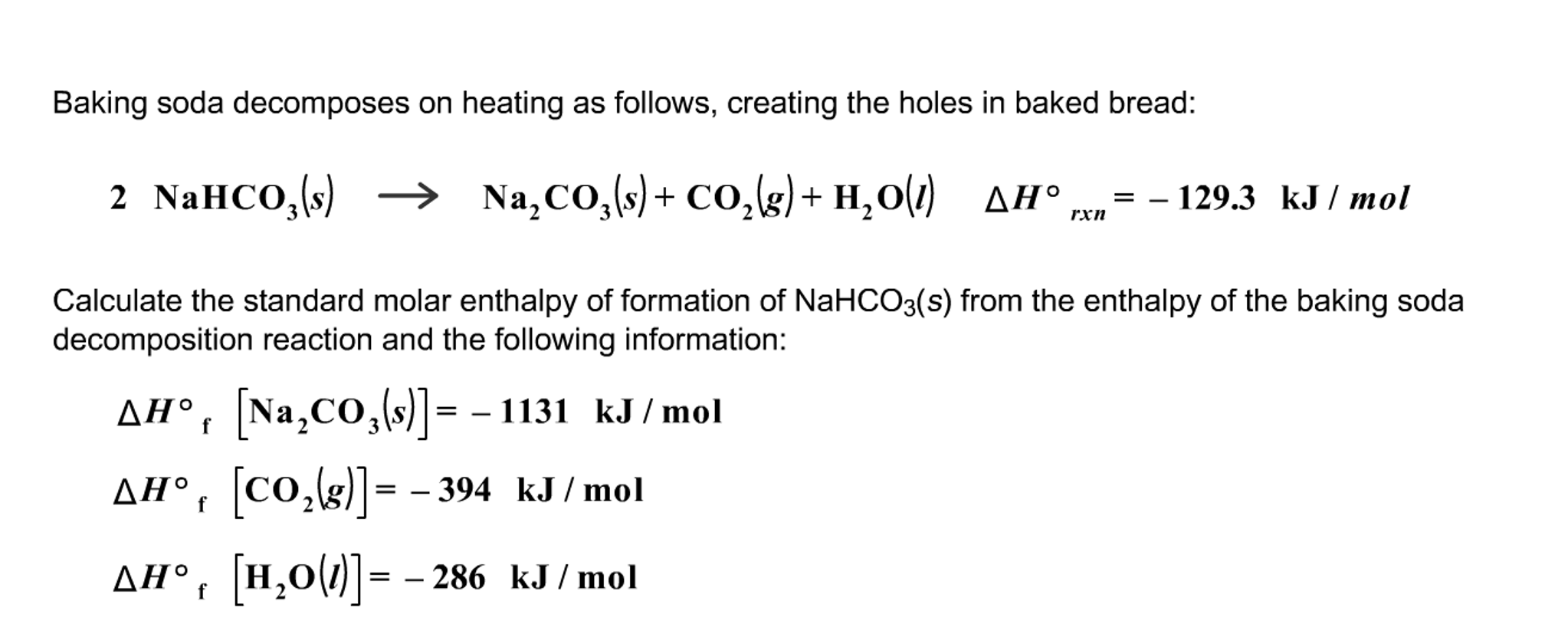
SOLVED: Baking soda decomposes on heating as follows: 2NaHCO3(s) â†' Na2CO3(s) + CO2(g) + H2O(l) ΔHrxn = -129.3 kJ. Calculate the standard enthalpy of formation of NaHCO3(s): Show your work for credit.
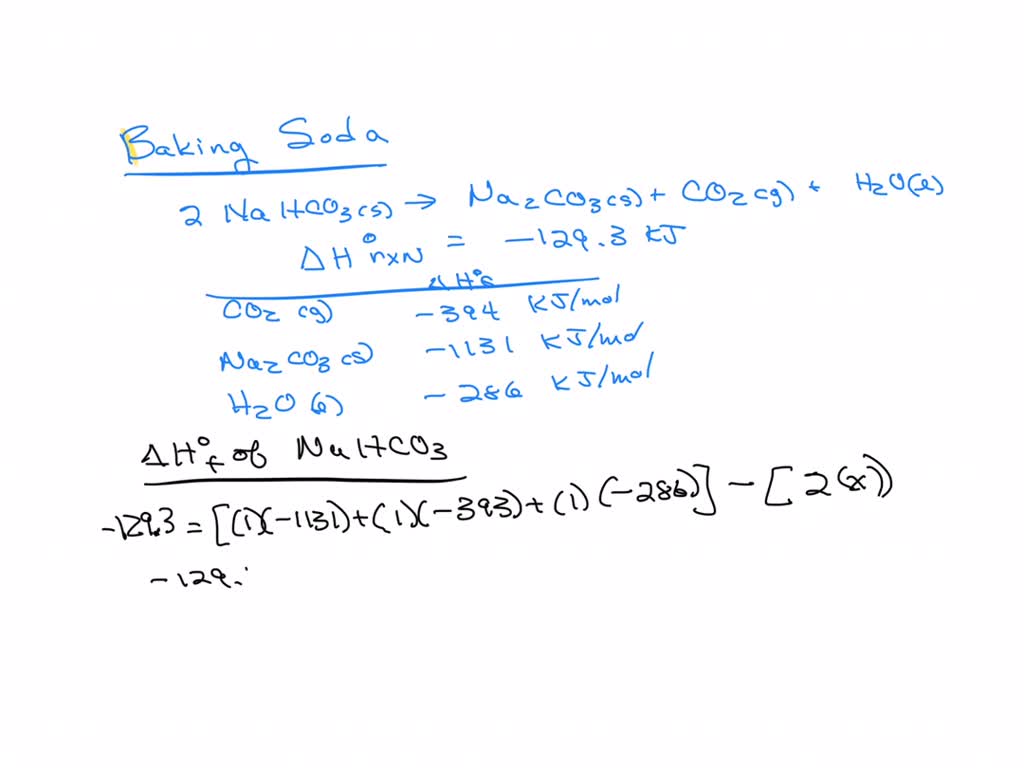
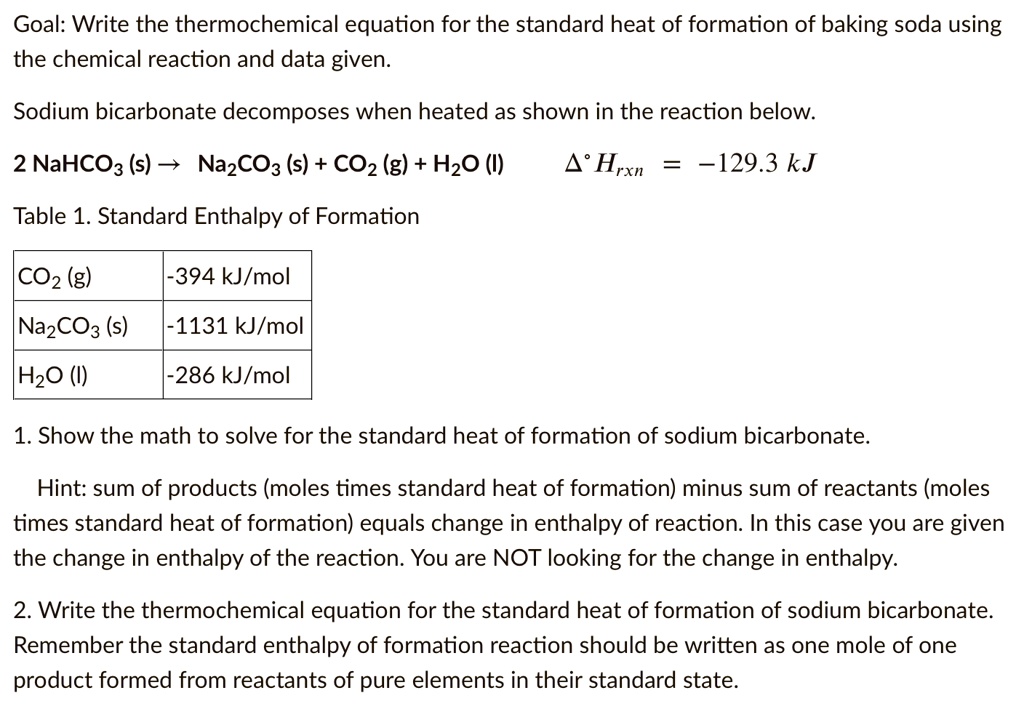
SOLVED: Goal: Write the thermochemical equation for the standard heat of formation of baking soda using the chemical reaction and data given. Sodium bicarbonate decomposes when heated as shown in the reaction

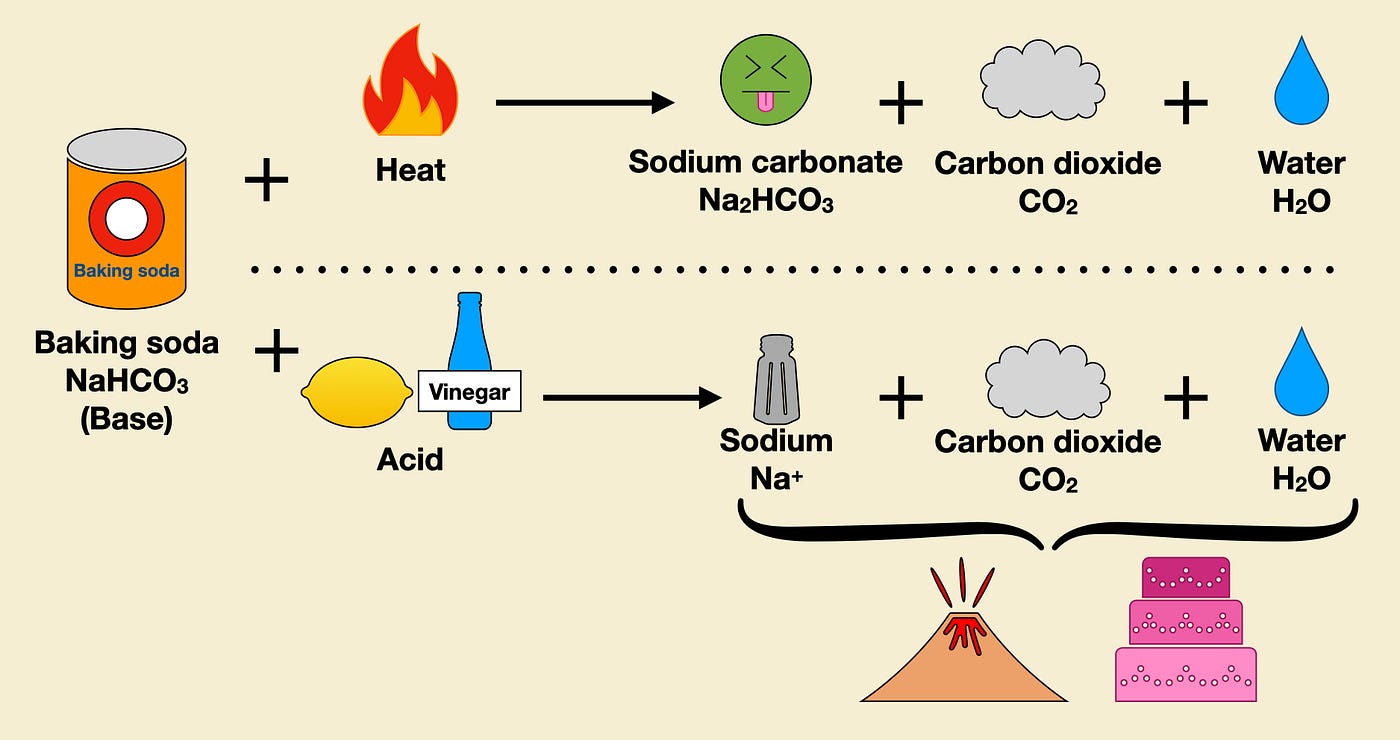
what happen when a solution of baking soda is heated write chemical equation for the same and name the - Brainly.in

A hydrated solid X on heating initially gives a monohydrated compound Y. Y upon heating above 373 K leads to an anhydrous white powder Z. X and Z, respectively, are: